General Duties
Duties of
the ASF-EURL
The European Council and the Commission have designated European Union Reference Laboratories (EURLs) with scientific and technical expertise within the areas of:

in a number of legal acts to prevent, control and eradicate major animal diseases.
The EURL for African Swine Fever (ASF) was initailly set up under Council Directive 2002/60/EC in the Centro de Investigación en Sanidad Animal- Animal Health Research Centre- (CISA)-Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), belonging to Consejo Superior de Investigaciones Científicas (CSIC).
European Union reference laboratories designated in accordance with Article 93(1) shall be responsible for the following tasks insofar as they are included in the reference laboratories’ annual or multiannual work programmes that have been established in conformity with the objectives and priorities of the relevant work programmes adopted by the Commission in accordance with Article 16 of Regulation (EU) No 2021/690: (taking into account Art 147 of (EU) 625/2017).
The EURL for ASF is accredited to ISO/IEC 17025 on “General requirements for the competence of testing and calibration laboratories by the Spanish Accreditation Service (ENAC).
The duties of the EURL, in line with Article 94 of the ‘Official Control Regulation’ for ASF are:

providing national reference laboratories with details and guidance on the methods of laboratory analysis, testing or diagnosis, including reference methods;

providing reference materials to national reference laboratories;

coordinating the application by the national reference laboratories and, if necessary, by other official laboratories of the methods referred to in point (a), in particular, by organising regular inter-laboratory comparative testing or proficiency tests and by ensuring appropriate follow-up of such comparative testing or proficiency tests in accordance, where available, with internationally accepted protocols, and informing the Commission and the Member States of the results and follow-up to the inter-laboratory comparative testing or proficiency tests;

coordinating practical arrangements necessary to apply new methods of laboratory analysis, testing or diagnosis, and informing national reference laboratories of advances in this field;

conducting training courses for staff from national reference laboratories and, if needed, from other official laboratories, as well as of experts from third countries;

providing scientific and technical assistance to the Commission within the scope of their mission;

providing information on relevant national, Union and international research activities to national reference laboratories;

collaborating within the scope of their mission with laboratories in third countries and with the European Food Safety Authority (EFSA), the European Medicines Agency (EMA) and the European Centre for Disease Prevention and Control (ECDC);

assisting actively in the diagnosis of outbreaks in Member States by carrying out confirmatory diagnosis, characterisation and taxonomic or epizootic studies on pathogen isolates or pest specimens;

coordinating or performing tests for the verification of the quality of reagents and lots of reagents used for the diagnosis;

where relevant for their area of competence, establishing and maintaining: i) reference strains; and ii) up-to-date lists of available reference substances and reagents and of manufacturers and suppliers of such substances and reagents; and

where relevant for their area of competence, cooperate among themselves and with the Commission, as appropriate, to develop methods of analysis, testing or diagnosis of high standards.
As regards point (i) of point (k), the EURL may establish and maintain those reference collections and reference strains by contractual outsourcing to other official laboratories and to scientific organisations.
The EURL shall publish the list of the national reference laboratories designated by the Member States in accordance with Article 100(1) of Regulation (EU) 2016/429
CURRENT ACTIVITIES
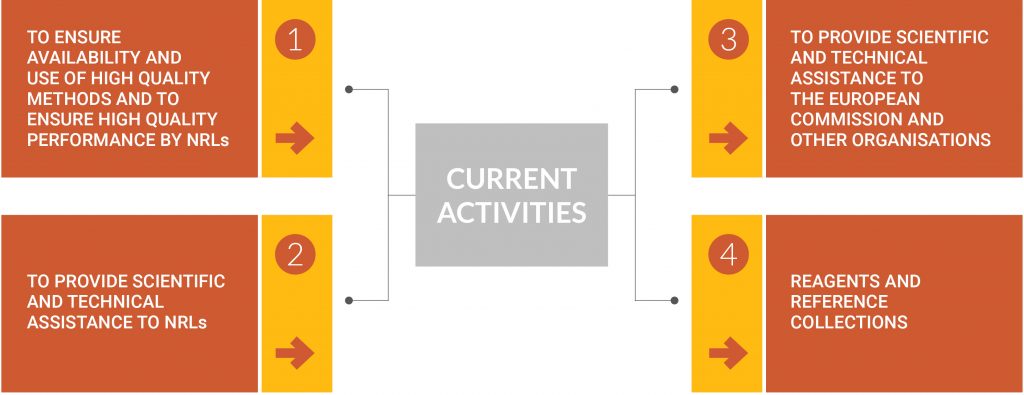
ACTIVITY 1. TO ENSURE AVAILABILITY AND USE OF HIGH QUALITY METHODS AND TO ENSURE HIGH QUALITY PERFORMANCE BY NRLs, by means of:
- Sub-activity 1.1: Development and/or update of the guidelines or Standard Operating Procedures (SOPs) of the diagnostic methods and diagnosis conclusion.
- Sub-activity 1.2: Preparation, control and supply of ASF reference standards, biological material and reagents for ASF diagnosis.
- Sub-activity 1.3: Preparation, control and supply of ASF-EURL reference material for validation and internal verification of ASF diagnostic techniques.
- Sub-activity 1.4. Organization of the annual Inter-Laboratory comparison test (ILCT) for ASF. Evaluation of the results obtained from the different laboratories during the Inter-Laboratory comparison tests for ASF (ILCTs-ASF).
ACTIVITY 2. TO PROVIDE SCIENTIFIC AND TECHNICAL ASSISTANCE TO NRLs consisting of
- Sub-activity 2.1: Conducting face to face and/or virtual training courses on ASF laboratory diagnosis for staff from NRLs and, if needed, from other official laboratories, as well as of experts from third countries at the EURL.
- Sub-activity 2.2: training workshop on ASF diagnosis for staff from NRLs from EU countries and other NRL collaborating European countries at the EURL, after a request.
- Sub-activity 2.3: Organization -in collaboration with the EURL for Classical Swine Fever (CSF) of the “Workshop on Laboratory Diagnosis of African and Classical Swine Fever (ASF and CSF)”.
- Sub-activity 2.4: Assisting EU NRLs and other official laboratories from EU candidate MS as well as neighbouring countries with the EU, in particular in affected and/or high-risk European countries, through EURL Missions on the spot, previous request.
- Sub-activity 2.5: Maintenance and update of the EURL web site and sequence data bank.
ACTIVITY 3. TO PROVIDE SCIENTIFIC AND TECHNICAL ASSISTANCE TO THE EUROPEAN COMMISSION AND OTHER ORGANISATIONS through
- Sub-activity 3.1: To have available qualified EURL staff to assist during outbreaks / crisis situations and/or to engage the EURL on-site.
- Sub-activity 3.2: Participation in webinars or meetings within the EU and European countries due to emergency and /or high-risk situation. Active participation in ASF global platforms and expert´s webinars.
- Sub-activity 3.3: Active assistance to MS in the diagnosis of ASF outbreaks, and other EU neighbouring countries if requested/needed.
ACTIVITY 4. REAGENTS AND REFERENCE COLLECTIONS
- Sub-activity 4.1: Verification of the quality of reagents and batch of reagents used for the diagnosis.
- Sub-activity 4.2: Characterization of viral isolates collected in the ASF outbreaks worldwide for the maintenance and updating of the ASF virus and sera banks.
